Best Describes the Bonding Character of This Ion
It is one of the main types of bonding along with covalent bonding and metallic bonding. What is ionic character and electronegativity.
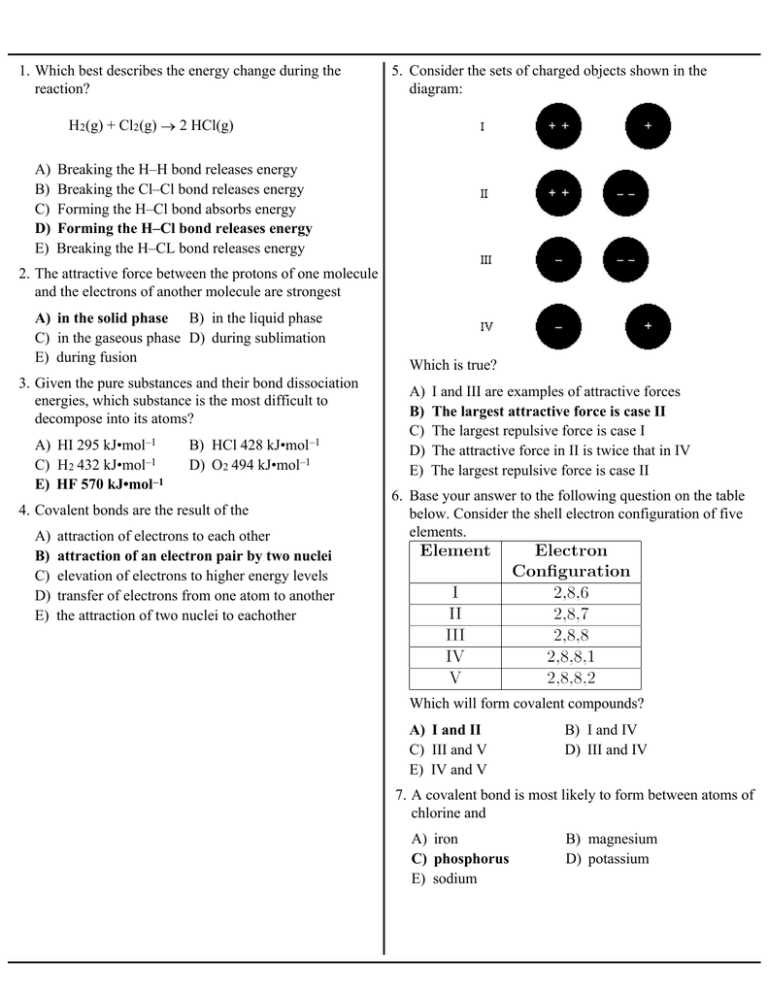
A Breaking The H H Bond Releases Energy B Breaking The Cl Cl
The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds.
. A The forming of the H-Cl bond releases energy. Ions are atoms with an electrostatic charge. 32 Explain in terms of electronegativity why an H-F bond is expected to be more polar than an H-I bond.
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities and is the primary interaction occurring in ionic compounds. Explanation of Kossel Lewis Approach. Which statement is true about a polyatomic ion.
Ionic Character Trend. One way of estimating the ionic character of a bondthat is the magnitude of the charge separation in a polar covalent bondis to calculate the difference in electronegativity between the two atoms. B mainly covalent with an increasing tendency towards ionic as you go up the group.
Each 33 Given the reaction. Drawing the Lewis Structure for HCO 2-. Which of the following best describes the shape of SO3 molecules.
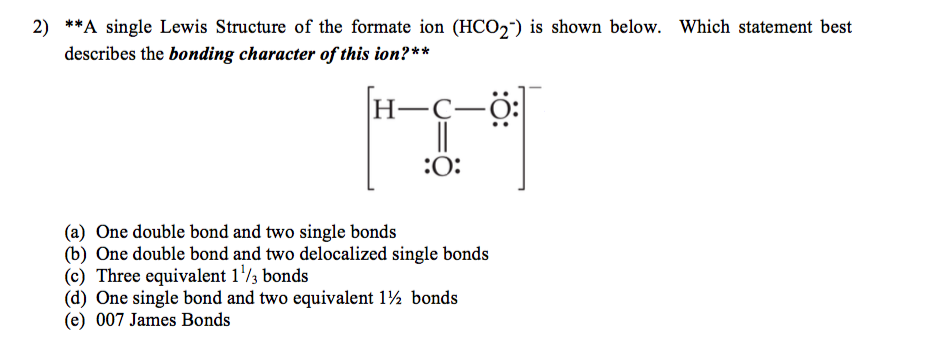
HCO 2-is also called Formate Ion. Which statement best describes the bonding character of this ion. The partial sharing of electrons between atoms that have an ionic bond.
Ionic bonding is a chemical bond. BOH 2 1 2Bonding. A linear b square planar c tetrahedral.
Each hydrogen atom contributes one 1s atomic orbital and thus the orbitals overlap according to MO theory to form one σ1s and one σ 1s MO by conservation of orbitals. Diethyl ether contains an oxygen atom that is a hydrogen bond acceptor because it is not bonded to a hydrogen atom and so is. Before looking at the factors that influence the formation of ionic bonds lets look at basic definitions.
Each hydrogen atom contributes one electron and thus H 2 has three electrons while H 2 has one. The electrons are donated to the B in the BF3. The covalent bond is a bond formed when two atoms share one or more electron pairs.
This kind of chemical bonding existing between two unlike charged particles is known as an electrovalent bond. Which statement best describes the bonding character of this ion. B Ionic bonds result from the transfer of electrons from a metal to a non-metal.
A Covalent bonds result from the transfer of electrons from one element to. D The ion has a trigonal pyramidal shape. The extent to which two or more elements share electrons in a bond is known as ionic character.
So there is a partial polarity created. Strong single covalent bonds with weak intermolecular forces. Remember that the ionic character of the bond is more than 50.
A The ion has a tetrahedral shape. 2 pts BONUS Questions 1 pt. A lattice of positive and negative ions held together by electrostatic forces.
C mainly ionic with an increasing tendency towards covalent as you go down the group. Which of the following has the greatest covalent character. The stronger the ionic bond in a cell the weaker the cell is because this signifies that there may be water available and water can make bonds weaker.
They are made up of particles that are arranged in a repeating pattern. Electronegativity can be used to determine the ionic character of a chemical bond. Δχ χ B χ A.
Strong multiple covalent bonds including pi bonds with weak intermolecular forces. It is made of atoms that are covalently bonded together. Ionic character of a covalent bond is when the electro negativity of the two atoms bonding differs.
D Covalent bonds result from the sharing of electrons between two metals. A covalent bond that has a partial ionic character to it as a result of the difference in electronegativity between the two bonding atoms. Ethanol contains a hydrogen atom that is a hydrogen bond donor because it is bonded to an electronegative oxygen atom which is very electronegative so the hydrogen atom is slightly positive.
When a covalent bond forms the F- ion donates both of the bonding pairs of electrons. Each atom contributes an equal number of electrons towards the bond formation. There are a total of 18 valence electrons in the HCO 2-Lewis structure.
Hydrogen bond donor and hydrogen bond acceptor. This bond is H to F with an electronegativity of 19. D 2 s p 3 hybridized orbitals of F e 2 are 6 electron pairs are from C N ion occupy the six hybrid d 2 s p 3 orbitals.
Since there are six ligands around the central metal ion the most feasible hybridization is d 2 s p 3. B The H-O-H bond angle is 120. A single Lewis Structure of the formate ion HCO2 is shown below.
If you calculate their bond order you get. 24 The bonding in gaseous hydrogen halides is best described as A mainly covalent with an increasing tendency towards ionic as you go down the group. H2 Cl 2 2HCl Which statement best describes the energy change as bonds are formed and broken in this reaction.
A One double bond and two single bonds b One double bond and two delocalized single bonds c Three equivalent 1 13 bonds d One single bond and two equivalent 1 12 bonds e 007 James Bonds. A measure of the tendency of an atom to attract electrons to itself. A NaCl b MgCl2 c AlCl3 d SiCl4.
Which statement is true about ionic compounds. With HCO 2-youll need to form a double bond between one of the Oxygen atoms and the Carbon atom to fill the octets. C Ionic bonds result from the sharing of electrons between two nonmetals.
Carbon C is the least electronegative atom and goes at the center of the HCO 2-Lewis structure. When two different atoms with different electronegative values form a covalent bond the atom with a higher electronegative value tends to draw the bonding electrons towards itself. Using a periodic table seen in the previous section elements which tend to.
The structure that best describes the double bond character of the amide bond in acetamide is shown by the Roman numeral _____ asked Jun 15 2017. For example MgCl2 the magnesium ion and chlorine ions are held together by force of electrostatic attraction. Which of the following best describes the type of bonding in a sample of CO2 s answer choices.
C The H-O-H bond angle is 90. O 007 James Bonds O Three equivalent 13 bonds o One double bond and two delocalized single bonds o One single bond and two equivalent 112 bonds o One double bond and two single bonds. A single Lewis Structure of the formate ion HCO-_2 is shown below.

Solved A Single Lewis Structure Of The Formate Ion Hco 2 Chegg Com
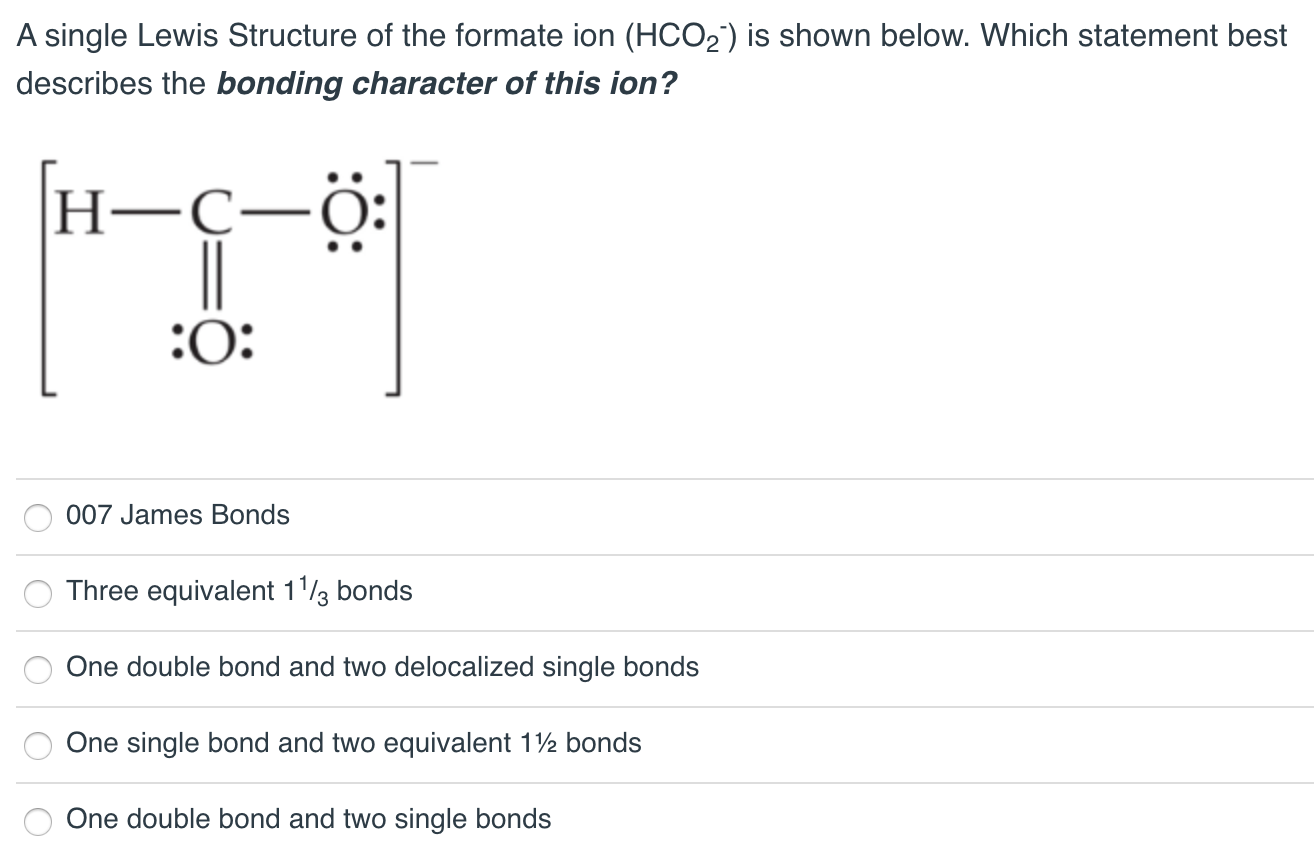
Solved A Single Lewis Structure Of The Formate Ion Hco2 Is Chegg Com

No comments for "Best Describes the Bonding Character of This Ion"
Post a Comment